Contact : +91-95601 02589
BATCH COMMENCEMENT
Hands on Training PSUR PBRER PADER DSUR Writing
11,500+ Cliniminds Alumni
BATCH COMMENCEMENT
Hands on Training
PSUR PBRER PADER DSUR Writing
11,500+ Cliniminds Alumni
Certifications
-
Cliniminds – ACCRE, USA Accredited.
Program Details – Modules
- Aggregate Reports, Their significance and Types of Reports.
- Preparation and distribution of Periodic Safety Update Reports (PSURs).
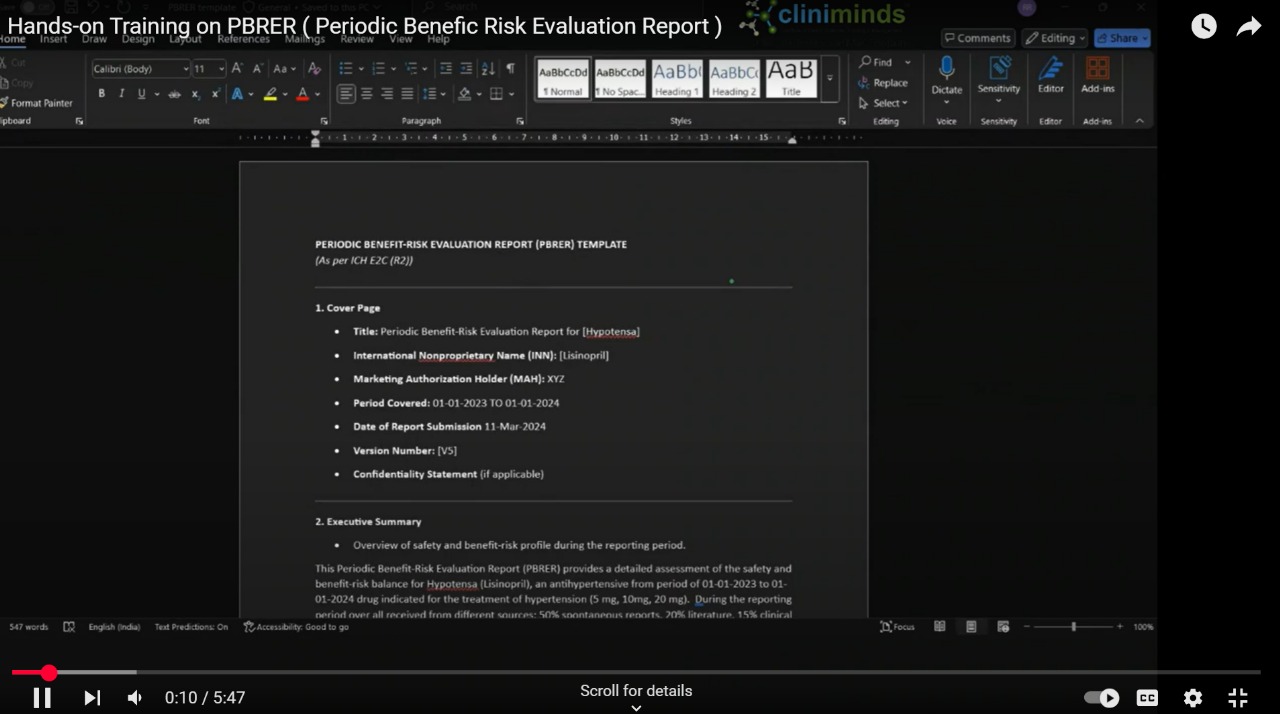
- Preparation and distribution of Periodic Benefit- Risk Evaluation Reports(PBRERs).
- Signal detection of Periodic Benefit- Risk Evaluation Reports (PBRERs).
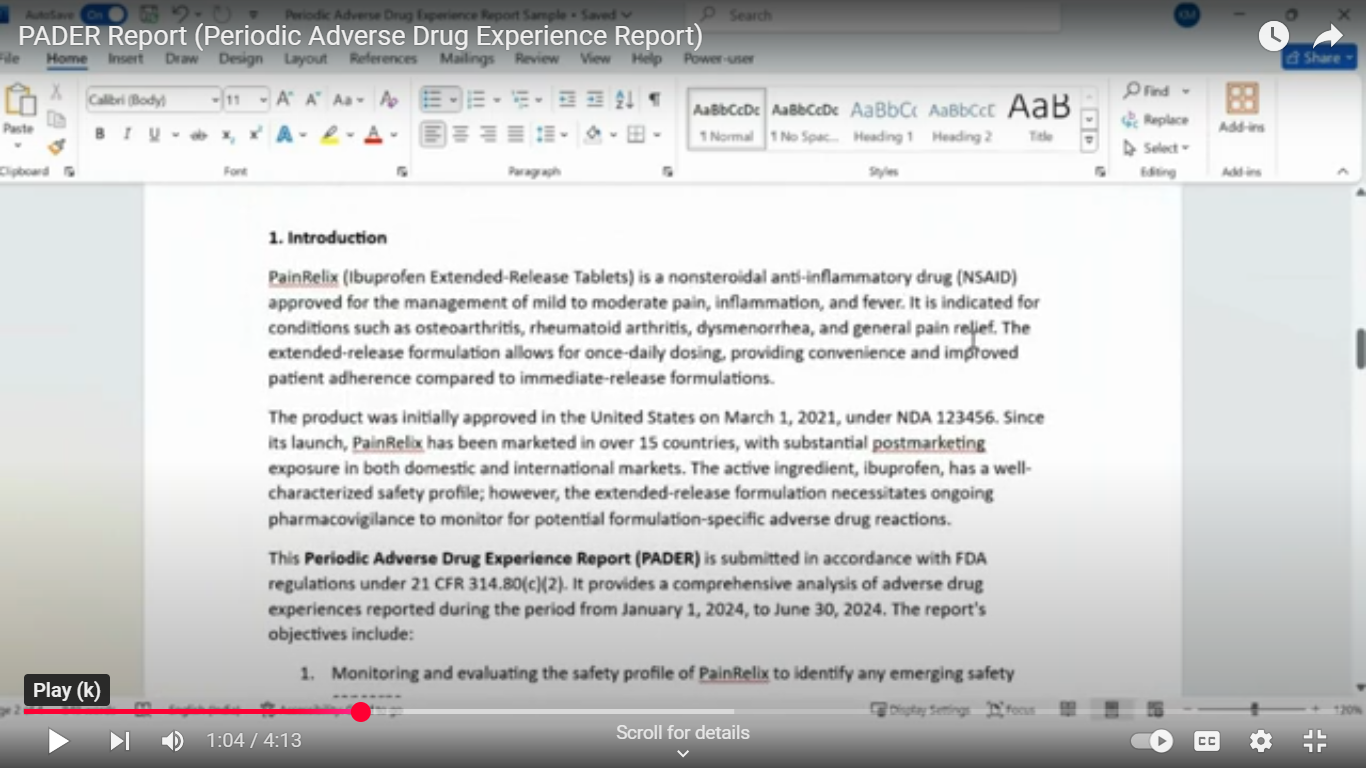
- Preparation and distribution of Periodic Adverse Drug Experience Reports(PADERs).
- Preparation and distribution of Developmental Safety Update Report(DSURs).
- Reference Safety Information – Selection & Preparation.
Program Inclusions
- Live Audio Video eLearning Sessions.
- Hands on Training on Preparing Aggregate Reports – PSUR, DSUR, PBRER, PADER.
- Line Listing Extraction Training.
- Access to Cliniminds LMS 24x7 – Session Recordings, PPT, Notes.
- Cliniminds Certification – USA ACCRE Accredited.
Certifications
-
Cliniminds – ACCRE, USA Accredited.
Program Details – Modules
- Aggregate Reports, Their significance and Types of Reports.
- Preparation and distribution of Periodic Safety Update Reports (PSURs).
- Preparation and distribution of Periodic Benefit- Risk Evaluation Reports(PBRERs).
- Signal detection of Periodic Benefit- Risk Evaluation Reports (PBRERs).
- Preparation and distribution of Periodic Adverse Drug Experience Reports(PADERs).
- Preparation and distribution of Developmental Safety Update Report(DSURs).
- Reference Safety Information – Selection & Preparation.
Program Inclusions
- Live Audio Video eLearning Sessions.
- Hands on Training on Preparing Aggregate Reports – PSUR, DSUR, PBRER, PADER.
- Line Listing Extraction Training.
- Access to Cliniminds LMS 24x7 – Session Recordings, PPT, Notes.
- Cliniminds Certification – USA ACCRE Accredited.
ADVANCED PHARMACOVIGILANCE & DRUG SAFETY PROGRAM FOR FAST CHANGING PHARMACOVIGILANCE SECTOR
Pharmacovigilance business is going through major shift due to constantly changing regulatory environment, other emerging locations and advent of Artificial Intelligence. Though India continues to be a major global hub for pharmacovigilance, other destinations are emerging as alternatives to India due to availability of skilled workforce. As a result, the job market is going through major structural changes. India must maintain its edge as a centre for top global skilled workforce.
Cliniminds team of recruiters spend significant time with various global pharmacovigilance experts for various pharmacovigilance consulting services and recruitment services. We interview several middle to senior management professionals for various positions in different geographies every day. This puts us in a unique position to understand the changing marketplace.
To remain relevant in this fast-changing environment, it is very important to learn multiple skill sets in the pharmacovigilance domain. Some of the important areas we recommend are:
- Aggregate Report Writing
- Signal Detection and Risk Management
- Agreements, Contracts and Essential Pharmacovigilance Documents
- PSMF
- PQMS
- Commercial and Business Contracts
Cliniminds is a global leader in pharmacovigilance education, training, and recruitment since 2004. Trained over 6000+ Pharmacovigilance professionals globally.
Cliniminds offers Post Graduate Diploma in Advanced Pharmacovigilance & Drug Safety Management. This program is designed for experienced pharmacovigilance professionals with 2+ years of experience in case processing or medical review.
This program would prepare you for the fast-changing global pharmacovigilance industry.
Training Methodology
The program consists of live eLearning interactive sessions with senior industry experts, eBooks, presentations, audio-video recordings, and hands-on training on various reports and processes. At the end of the program, there would be multiple sessions to prepare you for various job roles and CV writing.